Computational and Structural Biotechnology Journal; Volume 19, 2021, Pages 4192-4206
https://doi.org/10.1016/j.csbj.2021.07.019
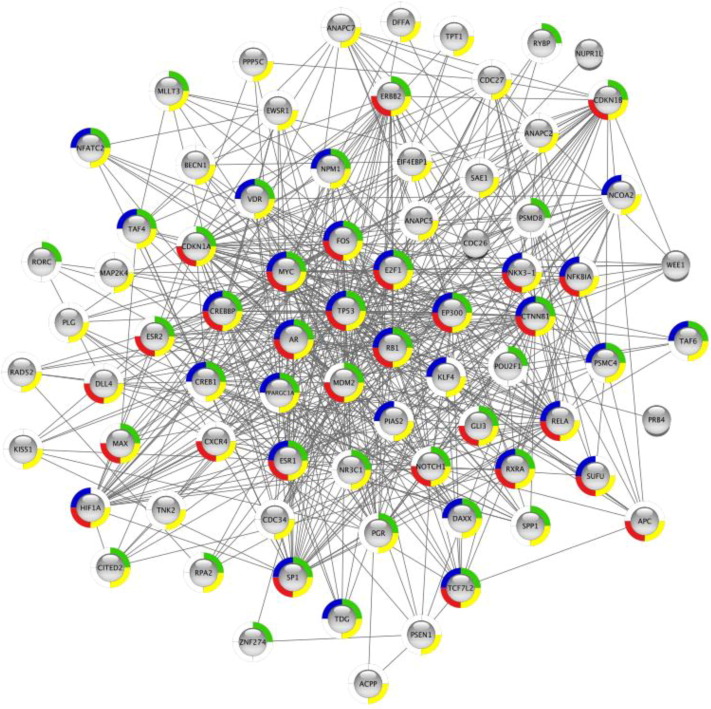
Abstract
The amyloid conformation is considered a fundamental state of proteins and the propensity to populate it a generic property of polypeptides. Multiple proteome-wide analyses addressed the presence of amyloidogenic regions in proteins, nurturing our understanding of their nature and biological implications. However, these analyses focused on highly aggregation-prone and hydrophobic stretches that are only marginally found in intrinsically disordered regions (IDRs). Here, we explore the prevalence of cryptic amyloidogenic regions (CARs) of polar nature in IDRs. CARs are widespread in IDRs and associated with IDPs function, with particular involvement in protein–protein interactions, but their presence is also connected to a risk of malfunction. By exploring this function/malfunction dichotomy, we speculate that ancestral CARs might have evolved into functional interacting regions playing a significant role in protein evolution at the origins of life.
