https://doi.org/10.1016/j.jsb.2020.107604
Highlights
- •
-
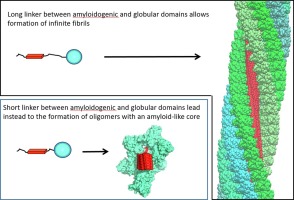
Globular domains flanking amyloid-forming regions can prevent the formation of cross-β amyloids.
- •
-
Coarse-grained and rigid body simulation show that these proteins form cross-β oligomers.
- •
-
The oligomerisation may provide insight into phase separation of proteins with amyloidogenic regions.
- •
-
This study opens up opportunities for design of nano-oligomers with functional domains.
Abstract
Insoluble amyloid fibrils formed by self-assembly of amyloidogenic regions of proteins have a cross-β-structure. In this work, by using targeted molecular dynamics and rigid body simulation, we demonstrate that if a protein consists of an amyloidogenic region and a globular domain(s) and if the linker between them is short enough, such molecules cannot assemble into amyloid fibrils, instead, they form oligomers with a defined and limited number of β-strands in the cross-β core. We show that this blockage of the amyloid growth is due to the steric repulsion of the globular structures linked to amyloidogenic regions. Furthermore, we establish a relationship between the linker length and the number of monomers in such nanoparticles. We hypothesise that such oligomerisation can be a yet unrecognised way to form natural protein complexes involved in biological processes. Our results can also be used in protein engineering for designing soluble nanoparticles carrying different functional domains.
