Our group works in the field of Theoretical Molecular Biology, encompassing a large path ranging from Theoretical Chemistry (at the borderline with Molecular Physics) to Biology (at the borderline with Medicine and Pharmacology). We use mainly Quantum Mechanics/Molecular Mechanics and Molecular Dynamics methods to carry out Biomolecular Simulations in silico for the study of enzyme activity: substrate binding, enzyme-substrate interactions, enzyme catalysis... The main purpose of our work is the understanding of these phenomena at a detailed molecular-level, and then the design/modification of the proteins/enzymes under study (using inhibitors, alosteric effects, mutations, radiation, electric fields or a combination of them) with the aim to control/modify their activity and/or function in a predefined direction, always with important biomedical and biotechnological applications. Progress in this direction can be relevant for the understanding and control, for instance, of inflammatory processes, in biocatalysis and in photopharmacology.
We not only apply the existing methods in Theoretical Chemistry, but we also develop other methods conveniently adapted to solve the challenges raised by the biological systems we study. Our purpose is to open new venues of experimental research starting from our theoretical results. We believe that Biomedicine and Theoretical Chemistry are connected disciplines that can work toguether to enhance the broad spectrum of human knowledge. With our research, we hope we can contribute to the rational design of new methods and drugs to act on human illnesses with as few undesirable secondary effects to human health as possible, thus, yielding contributions to practical advancements in Biomedicine and Pharmacology.
In the field of Biotechnology, we intend to design new enzymes as engineered biomolecular catalysts for the preparation of relevant organic molecules with better, faster, cheaper and with more selective synthetic processes than the ones currently available.
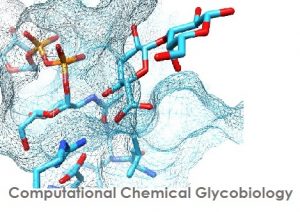
 We are interested on the study of carbohydrate-acting enzymes (CAZy) and of recognition processes involving glycans and carbohydrate derivatives:
We are interested on the study of carbohydrate-acting enzymes (CAZy) and of recognition processes involving glycans and carbohydrate derivatives:
- Reaction mechanism of CAZy enzymes.
- Enzyme engineering for the synthesis of glycans.
- Enzyme inhibition.
- Carbohydrate – lectin interactions
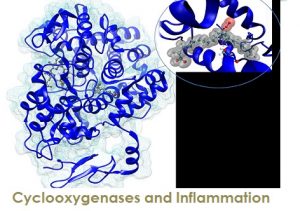
 Prostaglandin endoperoxide H synthase (PGHS) is a bifunctional hemoprotein endowed with both cyclooxygenase and peroxidase activities; since the former constitutes the first committed step on the biosynthetic pathway leading from arachidonic acid (AA) to prostaglandins (PGs), this enzyme is often referred to as cyclooxygenase (COX). Two isoforms of COX exist; while COX-1 is constitutive, the expression of COX-2 is induced during inflammatory states. PGG2 is the main product generated by COX upon dioxygenation of AA, with minor amounts of monooxygenated 11- and 15-hydroperoxyeicosatetraenoic (HPETE) acids. COX activity is substantially altered as a consequence of aspirin treatment. While acetylated COX-1 fails to generate any oxygenated products, in the case of COX-2 only PG generation is blocked, while the production of 11R- and 15-HETE is maintained. This switch in lipid mediator production by COX-2 has important therapeutic implications: metabolites of 15RHETE are endowed with potent pro-resolving activity, which sums up to the anti-inflammatory activity consequent to the blockade of PGG2 biosynthesis, making aspirin a unique, dual-acting irreversible COX inhibitor.
Prostaglandin endoperoxide H synthase (PGHS) is a bifunctional hemoprotein endowed with both cyclooxygenase and peroxidase activities; since the former constitutes the first committed step on the biosynthetic pathway leading from arachidonic acid (AA) to prostaglandins (PGs), this enzyme is often referred to as cyclooxygenase (COX). Two isoforms of COX exist; while COX-1 is constitutive, the expression of COX-2 is induced during inflammatory states. PGG2 is the main product generated by COX upon dioxygenation of AA, with minor amounts of monooxygenated 11- and 15-hydroperoxyeicosatetraenoic (HPETE) acids. COX activity is substantially altered as a consequence of aspirin treatment. While acetylated COX-1 fails to generate any oxygenated products, in the case of COX-2 only PG generation is blocked, while the production of 11R- and 15-HETE is maintained. This switch in lipid mediator production by COX-2 has important therapeutic implications: metabolites of 15RHETE are endowed with potent pro-resolving activity, which sums up to the anti-inflammatory activity consequent to the blockade of PGG2 biosynthesis, making aspirin a unique, dual-acting irreversible COX inhibitor.
Neither the mechanisms enforcing stereospecificity in AA oxygenation by native COX-2 nor the changes induced by aspirin treatment have been completely clarified so far. Our current goal is to reveal the molecular origin of aspirin effects by means of Molecular Dynamics and Quantum Mechanics/Molecular Mechanics calculations on the catalytic mechanism in wild type and acetylated COX-2 and COX-1, and the design of new inhibitors, especially molecular photoswitches with inhibiting properties of COX-2 specific to each isomer.
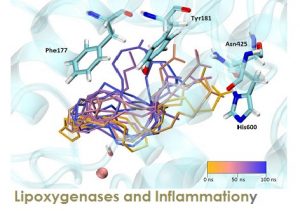
 Lipoxygenases (LOXs) form a family of lipid peroxidizing enzymes which have been implicated in a number of physiological processes and in the pathogenesis of inflammatory, hyperproliferative and neurodegenerative diseases. The LOX reaction constitutes a special type of lipid peroxidation and differs from non-enzymatic reactions in several respects, such as higher reaction rate, limited substrate selectivity, mechanisms of regulatory interference and the high product specificity. Non-enzymatic lipid peroxidation converts a given substrate to a complex array of primary oxygenation products whereas LOXs usually generate a single product isomer.
Lipoxygenases (LOXs) form a family of lipid peroxidizing enzymes which have been implicated in a number of physiological processes and in the pathogenesis of inflammatory, hyperproliferative and neurodegenerative diseases. The LOX reaction constitutes a special type of lipid peroxidation and differs from non-enzymatic reactions in several respects, such as higher reaction rate, limited substrate selectivity, mechanisms of regulatory interference and the high product specificity. Non-enzymatic lipid peroxidation converts a given substrate to a complex array of primary oxygenation products whereas LOXs usually generate a single product isomer.
Over the last years our group has been studying the catalytic mechanism of several LOX isoforms (rabbit ALOX15, pig ALOX15, coral ALOX15b, ALOX5) with linoleic and arachidonic acids as substrates by means of Docking, Molecular Dynamics and QM/MM calculations. Those studies have revealed the molecular origin of the exquisite regiospecificity of LOX catalysis that leads to the formation of inflammatory or anti-inflammatory agents. At present our main target is ALOX5, the most important human LOX, that catalyzes the hydroperoxidation of arachidonic acid leading either to the pro-inflammatory agents known as leukotrienes or, in combination with ALOX15, leading to the formation of lipoxins, anti-inflammatory molecules. In addition we will focus on the 5-lipoxygenase-activating protein (FLAP).