https://doi.org/10.1002/adma.201907348
Abstract
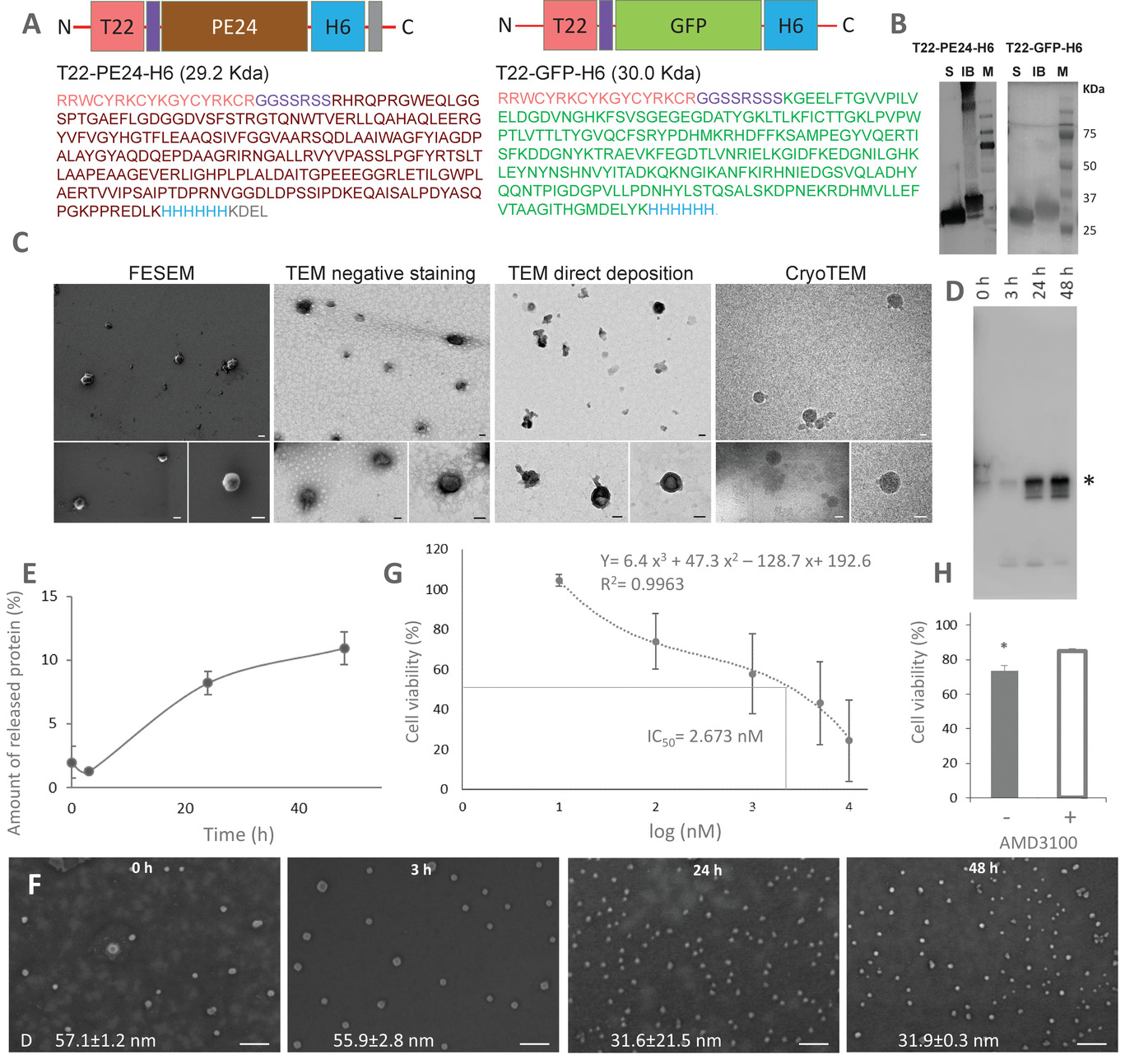
Functional amyloids produced in bacteria as nanoscale inclusion bodies are intriguing but poorly explored protein materials with wide therapeutic potential. Since they release functional polypeptides under physiological conditions, these materials can be potentially tailored as mimetic of secretory granules for slow systemic delivery of smart protein drugs. To explore this possibility, bacterial inclusion bodies formed by a self‐assembled, tumor‐targeted Pseudomonas exotoxin (PE24) are administered subcutaneously in mouse models of human metastatic colorectal cancer, for sustained secretion of tumor‐targeted therapeutic nanoparticles. These proteins are functionalized with a peptidic ligand of CXCR4, a chemokine receptor overexpressed in metastatic cancer stem cells that confers high selective cytotoxicity in vitro and in vivo. In the mouse models of human colorectal cancer, time‐deferred anticancer activity is detected after the subcutaneous deposition of 500 µg of PE24‐based amyloids, which promotes a dramatic arrest of tumor growth in the absence of side toxicity. In addition, long‐term prevention of lymphatic, hematogenous, and peritoneal metastases is achieved. These results reveal the biomedical potential and versatility of bacterial inclusion bodies as novel tunable secretory materials usable in delivery, and they also instruct how therapeutic proteins, even with high functional and structural complexity, can be packaged in this convenient format.
