Our group is interested in several topics concerning the biochemistry, the molecular biology and the genomics of the yeast Saccharomyces cerevisiae, specifically those that are related to cell signaling trough processes of phospho-dephosphorylation of proteins. For this purpose, we investigate issues such as ion homeostasis, the response to various stresses or the cell cycle regulation and how these circumstances affect specific protein kinases and/or phosphatases (activity, localization, binding to other proteins, postraductional modifications,…). The main objective is to obtain an overview on the yeast response to perturbations in its environment, so that we can both understand in deep the biology of this organism and to guide us towards new biotechnological applications or the identification of novel antifungal drugs targets
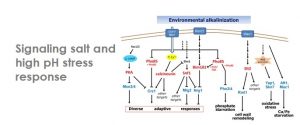

 Saline and high pH responses are widely regulated by phosphorylation mechanisms. We have characterized the role of diverse protein phosphatases, such as calcineurin, PP1, Ppz1, or members of the PP2C family in this processes by combination of classical and molecular genetics and biochemistry. In the case of high pH response, our group has been pivotal in the understanding of the complex signaling network at the basis of the strong pH-induced transcriptional remodeling, which involves protein kinases (such as PKA, Snf1, Slt2 or Pho85), protein phosphatases (such as calcineurin), and a multitude of transcription factors.
Saline and high pH responses are widely regulated by phosphorylation mechanisms. We have characterized the role of diverse protein phosphatases, such as calcineurin, PP1, Ppz1, or members of the PP2C family in this processes by combination of classical and molecular genetics and biochemistry. In the case of high pH response, our group has been pivotal in the understanding of the complex signaling network at the basis of the strong pH-induced transcriptional remodeling, which involves protein kinases (such as PKA, Snf1, Slt2 or Pho85), protein phosphatases (such as calcineurin), and a multitude of transcription factors.
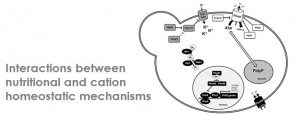
 Maintenance of proper gradients of protons and monovalent cations and uptake of nutrients are tightly linked in yeasts, and alteration in the former process affects the latter. We have investigated how changes in potassium availability or increase in the pH of the medium alters the uptake of diverse nutrients, from sugars to phosphate, forces remodeling of metabolic pathways, and growth rate and behavior of the yeast cells, promoting even invasive growth.
Maintenance of proper gradients of protons and monovalent cations and uptake of nutrients are tightly linked in yeasts, and alteration in the former process affects the latter. We have investigated how changes in potassium availability or increase in the pH of the medium alters the uptake of diverse nutrients, from sugars to phosphate, forces remodeling of metabolic pathways, and growth rate and behavior of the yeast cells, promoting even invasive growth.

Yeast Ppz1 is a type 1-like protein phosphatase that is found only in fungi, including pathogenic ones. In some cases, such as in C. albicans or A. fumigatus, ppz1 has been identified as a virulence factor. In S. cerevisiae this phosphatase is involved in several relevant functions such as the maintenance of cell wall integrity, cell-cycle regulation and the regulation of cation. It has been reported that, when overexpressed, Ppz1 is the most toxic yeast protein, pointing to the possibility that deregulation of Ppz1 activity in pathogenic fungi could become an antifungal drug target. We have demonstrated that Ppz1 toxicity derives from its phosphatase activity and is aggravated in less preferred carbon sources or under limiting glucose conditions. We are applying proteomics, transcriptomics, and classical genetic methods to elucidate the molecular basis of this toxicity in S. cerevisiae and in other pathogenic fungi.