Front. Bioeng. Biotechnol., 16 October 2020 | https://doi.org/10.3389/fbioe.2020.588947
Synucleinopathies are a group of disorders characterized by the accumulation of α-Synuclein amyloid inclusions in the brain. Preventing α-Synuclein aggregation is challenging because of the disordered nature of the protein and the stochastic nature of fibrillogenesis, but, at the same time, it is a promising approach for therapeutic intervention in these pathologies. A high-throughput screening initiative allowed us to discover ZPDm, the smallest active molecule in a library of more than 14.000 compounds. Although the ZPDm structure is highly related to that of the previously described ZPD-2 aggregation inhibitor, we show here that their mechanisms of action are entirely different. ZPDm inhibits the aggregation of wild-type, A30P, and H50Q α-Synuclein variants in vitro and interferes with α-Synuclein seeded aggregation in protein misfolding cyclic amplification assays. However, ZPDm distinctive feature is its strong potency to dismantle preformed α-Synuclein amyloid fibrils. Studies in a Caenorhabditis elegans model of Parkinson’s Disease, prove that these in vitro properties are translated into a significant reduction in the accumulation of α-Synuclein inclusions in ZPDm treated animals. Together with previous data, the present work illustrates how different chemical groups on top of a common molecular scaffold can result in divergent but complementary anti-amyloid activities.
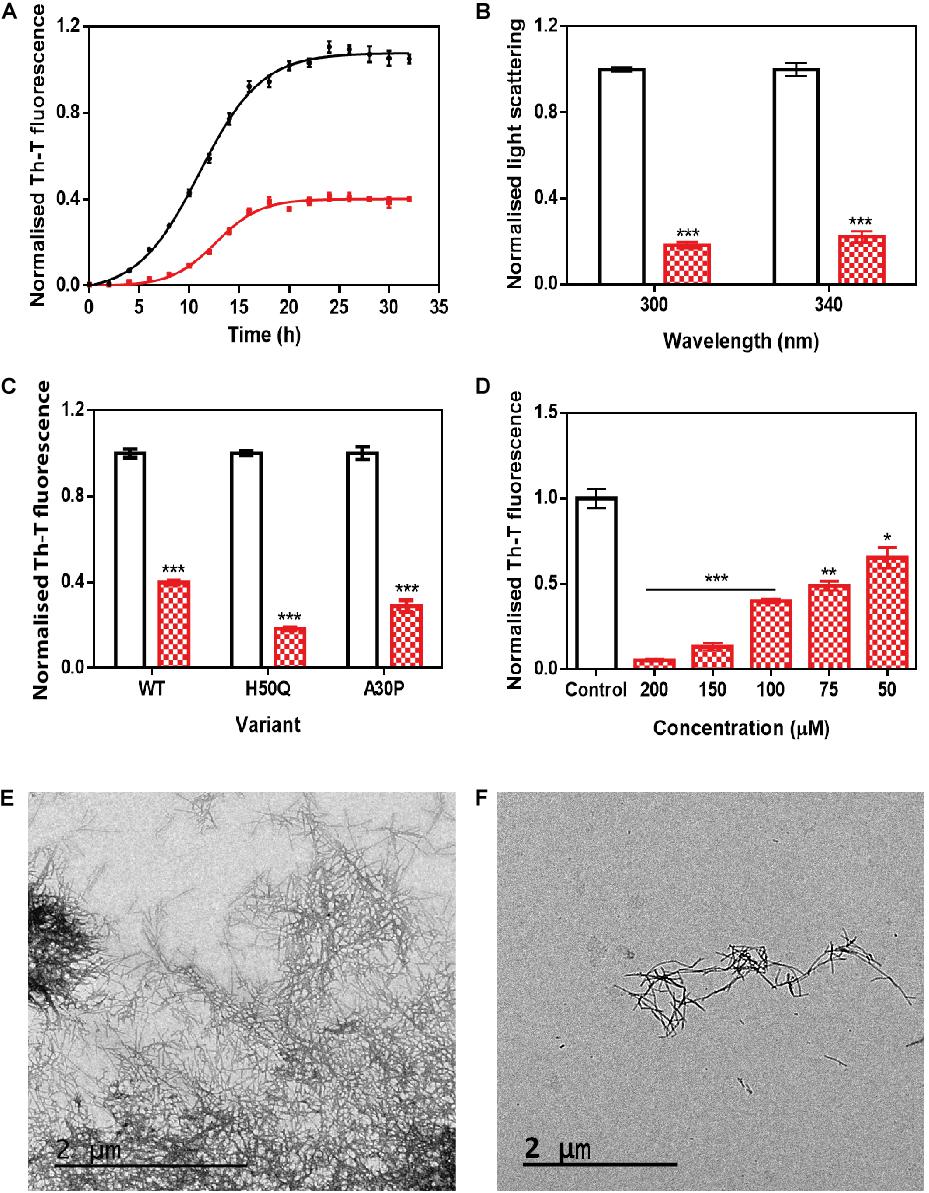
Figure 2. In vitro analysis of the capacity of ZPDm to inhibit α-Syn aggregation. (A) α-Syn aggregation kinetics in the absence (black) and presence (red) of 100 μM of ZPDm followed by Th-T fluorescence. (B) Light-scattering measurements at 300 and 340 nm, in the absence (white) and presence (red) of ZPDm. (C) H50Q and A30P α-Syn variants aggregation in the absence (white) and presence (blue) of ZPDm. (D) Inhibition of α-Syn aggregation with different concentrations of ZPDm. (E,F) Representative TEM images in the absence (E) and presence (F) of ZPDm. Th-T fluorescence is plotted as normalized means. Final points were obtained at 48 h. Error bars are represented as SE of mean values; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. ZPDm prevents the aggregation of WT, A30P, and H50Q α-Syn variants in vitro, even at substoichiometric ratios.
