doi:10.1016/j.bbamcr.2020.118727
Abstract
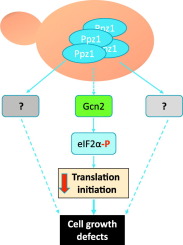
The Ser/Thr protein phosphatase Ppz1 from Saccharomyces cerevisiae is the best characterized member of a family of enzymes only found in fungi. Ppz1 is regulated in vivo by two inhibitory subunits, Hal3 and Vhs3, which are moonlighting proteins also involved in the decarboxylation of the 4-phosphopantothenoylcysteine (PPC) intermediate required for coenzyme A biosynthesis. It has been reported that, when overexpressed, Ppz1 is the most toxic protein in yeast. However, the reasons for such toxicity have not been elucidated. Here we show that the detrimental effect of excessive Ppz1 expression is due to an increase in its phosphatase activity and not to a plausible down-titration of the PPC decarboxylase components. We have identified several genes encoding ribosomal proteins and ribosome assembly factors as mild high-copy suppressors of the toxic Ppz1 effect. Ppz1 binds to ribosomes engaged in translation and copurifies with diverse ribosomal proteins and translation factors. Ppz1 overexpression results in Gcn2-dependent increased phosphorylation of eIF2α at Ser-51. Consistently, deletion of GCN2 partially suppresses the growth defect of a Ppz1 overexpressing strain. We propose that the deleterious effects of Ppz1 overexpression are in part due to alteration in normal protein synthesis.
Highlights
- •High catalytic activity of Ppz1 is detrimental for cell growth.
- •Overexpression of Ppz1 leads to Gcn2-dependent phosphorylation of eIF2α.
- •Ppz1 interacts with ribosomal components.
- •Excess of Ppz1 activity negatively affect the translation initiation process.
