https://www.cell.com/trends/molecular-medicine/fulltext/S1471-4914(20)30031-9
Highlights
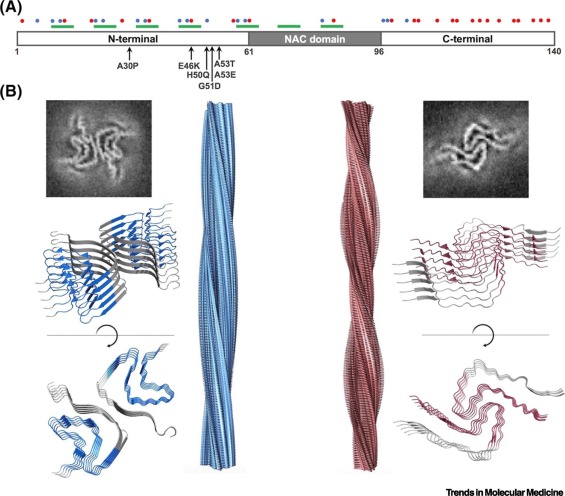
Parkinson’s disease (PD) is characterized by progressive loss of dopaminergic neurons and the accumulation of deposits of α-synuclein (α-syn) in the brain. The pivotal role of α-syn aggregation in PD makes it an attractive target for potential disease-modifying therapies. However, the disordered nature of the protein, its multistep aggregation mechanism, and the lack of structural information on intermediate species complicate the discovery of modulators of α-syn amyloid deposition. Despite these difficulties, small molecules have been shown to block the misfolding and aggregation of α-syn, and can even disentangle mature α-syn amyloid fibrils. In this review we provide an updated overview of these leading small compounds and discuss how these chemical chaperones hold great promise to alter the course of PD progression.