 https://elifesciences.org/articles/52978
https://elifesciences.org/articles/52978
Abstract
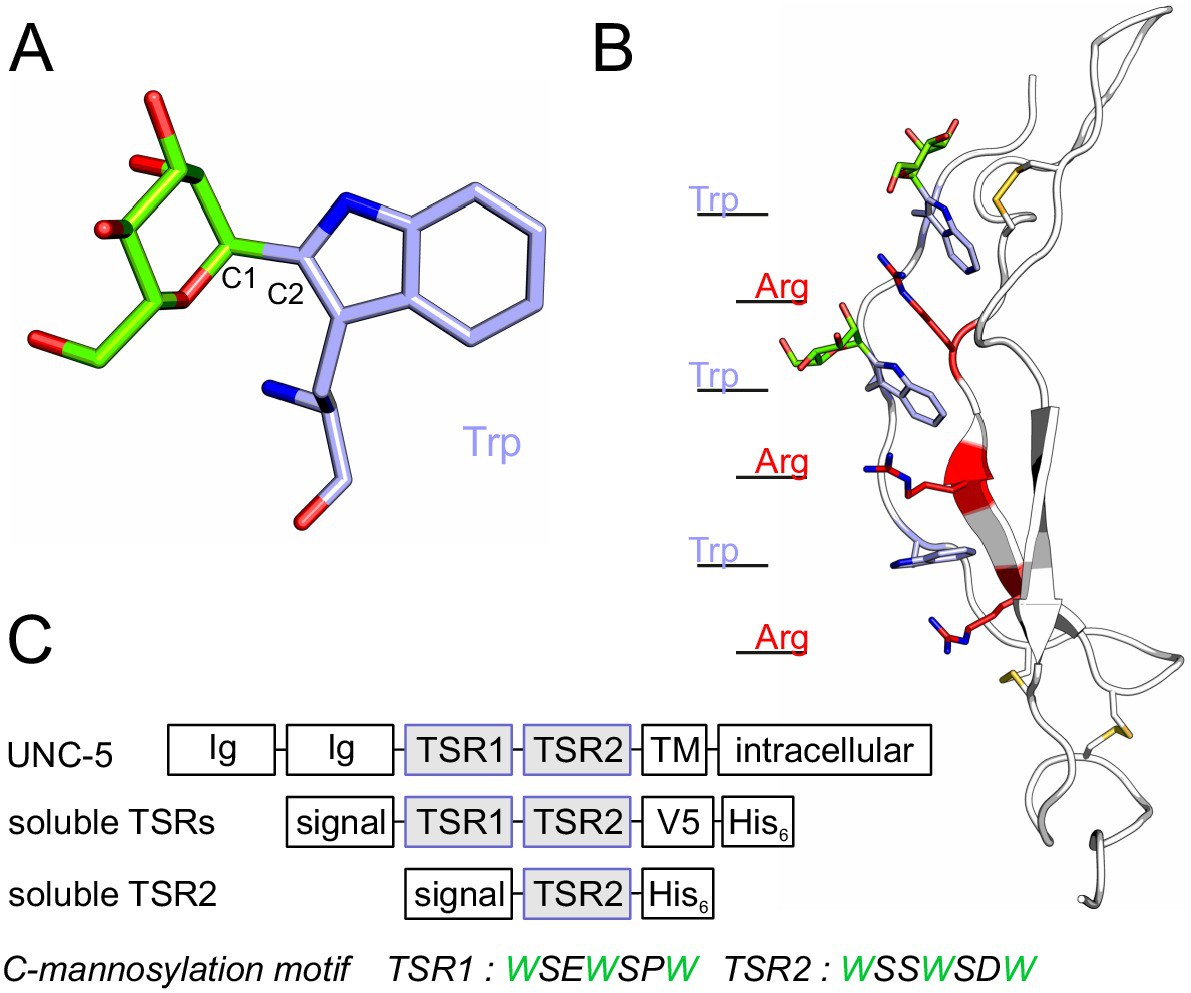
Previous studies demonstrated importance of C-mannosylation for efficient protein secretion. To study its impact on protein folding and stability, we analyzed both C-mannosylated and non-C-mannosylated thrombospondin type 1 repeats (TSRs) of netrin receptor UNC-5. In absence of C-mannosylation, UNC-5 TSRs could only be obtained at low temperature and a significant proportion displayed incorrect intermolecular disulfide bridging, which was hardly observed when C-mannosylated. Glycosylated TSRs exhibited higher resistance to thermal and reductive denaturation processes, and the presence of C-mannoses promoted the oxidative folding of a reduced and denatured TSR in vitro. Molecular dynamics simulations supported the experimental studies and showed that C-mannoses can be involved in intramolecular hydrogen bonding and limit the flexibility of the TSR tryptophan-arginine ladder. We propose that in the endoplasmic reticulum folding process, C-mannoses orient the underlying tryptophan residues and facilitate the formation of the tryptophan-arginine ladder, thereby influencing the positioning of cysteines and disulfide bridging.
