 Front. Mol. Neurosci., 17 December 2019 | https://doi.org/10.3389/fnmol.2019.00306
Front. Mol. Neurosci., 17 December 2019 | https://doi.org/10.3389/fnmol.2019.00306
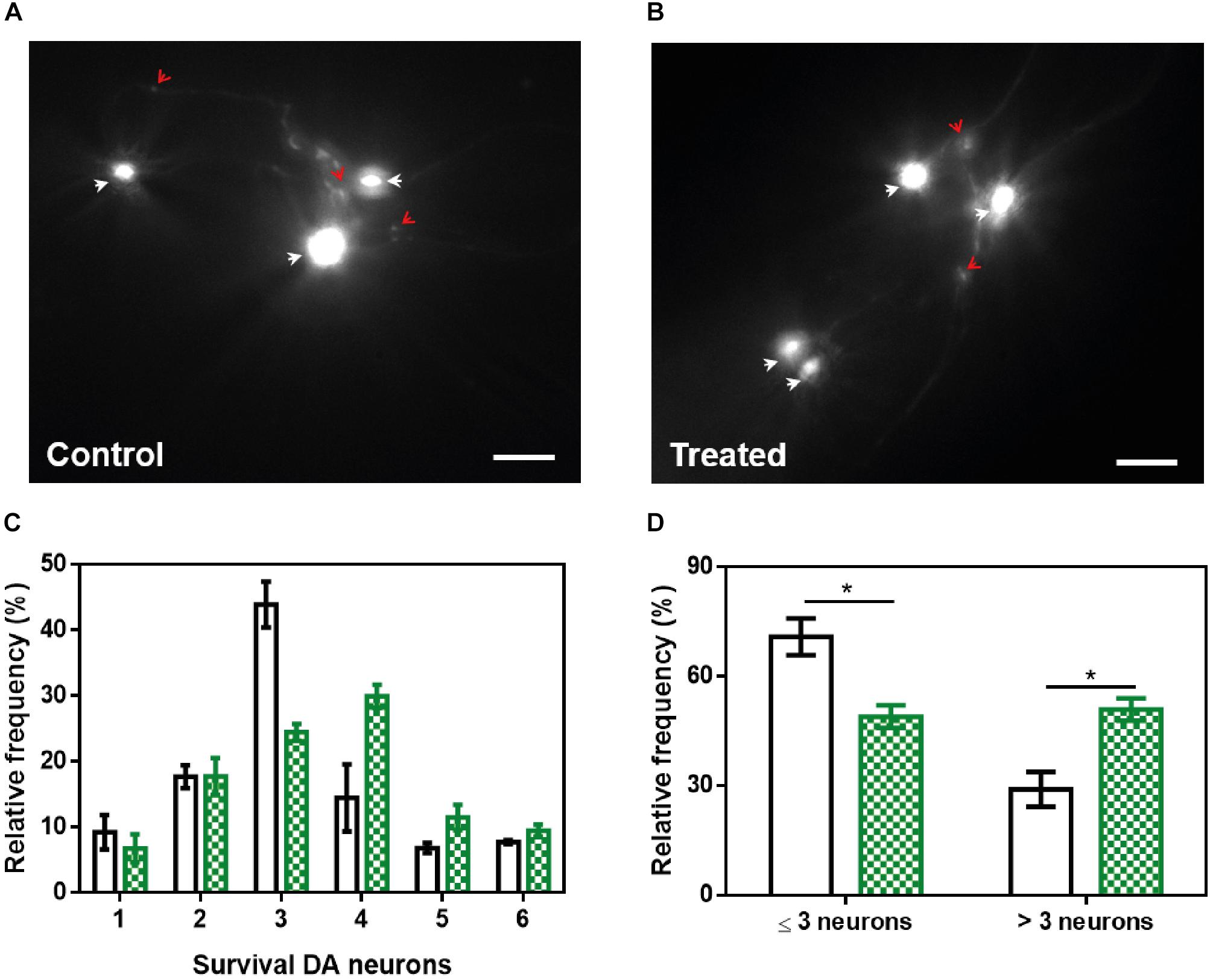
α-Synuclein (α-Syn) forms toxic intracellular protein inclusions and transmissible amyloid structures in Parkinson’s disease (PD). Preventing α-Syn self-assembly has become one of the most promising approaches in the search for disease-modifying treatments for this neurodegenerative disorder. Here, we describe the capacity of a small molecule (ZPD-2), identified after a high-throughput screening, to inhibit α-Syn aggregation. ZPD-2 inhibits the aggregation of wild-type α-Syn and the A30P and H50Q familial variants in vitro at substoichiometric compound:protein ratios. In addition, the molecule prevents the spreading of α-Syn seeds in protein misfolding cyclic amplification assays. ZPD-2 is active against different α-Syn strains and blocks their seeded polymerization. Treating with ZPD-2 two different PD Caenorhabditis elegans models that express α-Syn either in muscle or in dopaminergic (DA) neurons substantially reduces the number of α-Syn inclusions and decreases synuclein-induced DA neurons degeneration. Overall, ZPD-2 is a hit compound worth to be explored in order to develop lead molecules for therapeutic intervention in PD.
