SCIENCE CHINA Materials
Published online 19 September 2019 |
https://doi.org/10.1007/s40843-019-9582-9
http://engine.scichina.com/publisher/scp/journal/SCMs/doi/10.1007/s40843-019-9582-9?slug=fulltext
Abstract
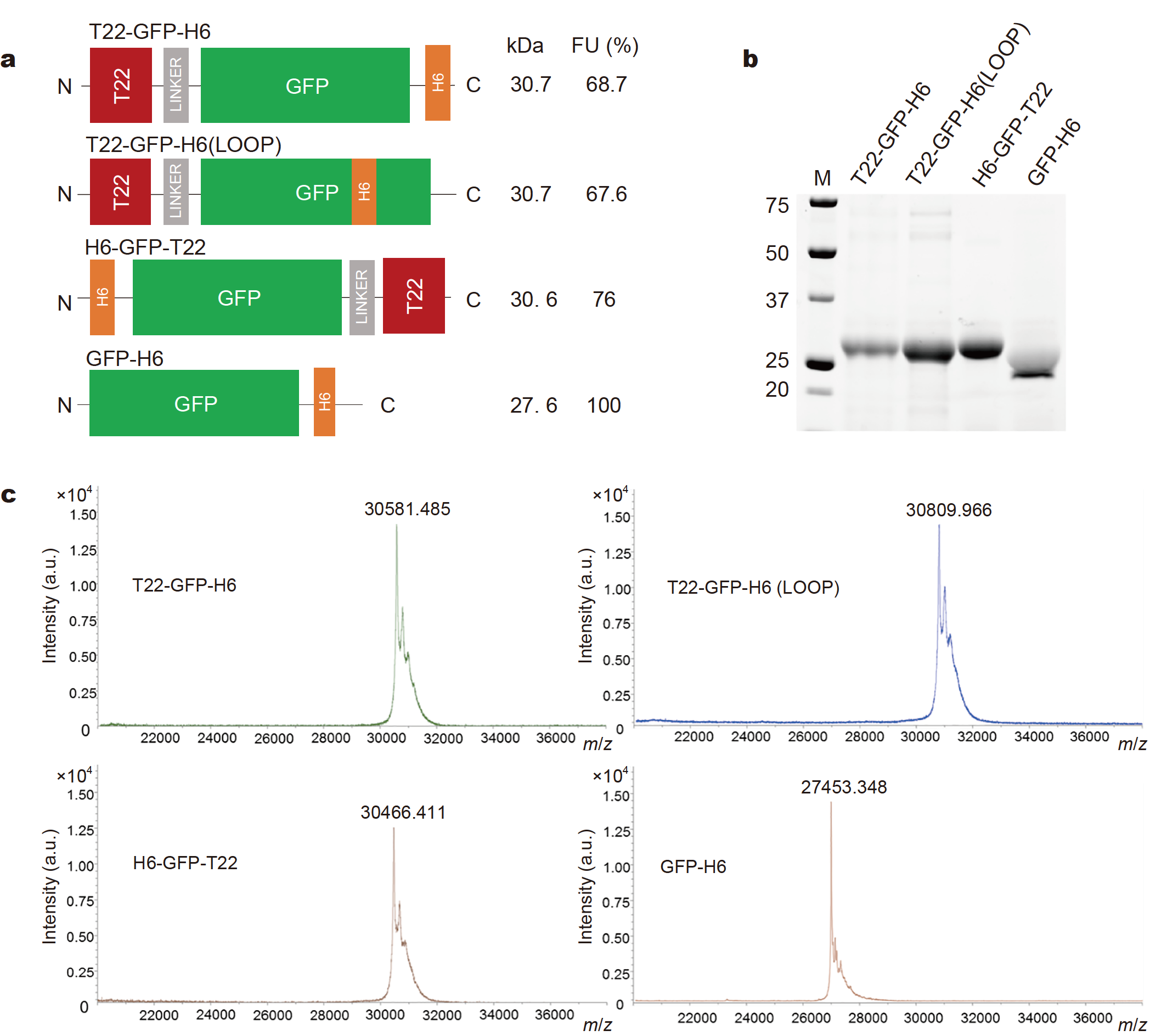
Modular protein engineering is suited to recruit complex and multiple functionalities in single-chain polypeptides. Although still unexplored in a systematic way, it is anticipated that the positioning of functional domains would impact and refine these activities, including the ability to organize as supramolecular entities and to generate multifunctional protein materials. To explore this concept, we have repositioned functional segments in the modular protein T22-GFP-H6 and characterized the resulting alternative fusions. In T22-GFP-H6, the combination of T22 and H6 promotes self-assembling as regular nanoparticles and selective binding and internalization of this material in CXCR4-overexpressing tumor cells, making them appealing as vehicles for selective drug delivery. The results show that the pleiotropic activities are dramatically affected in module-swapped constructs, proving the need of a carboxy terminal positioning of H6 for protein self-assembling, and the accommodation of T22 at the amino terminus as a requisite for CXCR4+ cell binding and internalization. Furthermore, the failure of self-assembling as regular oligomers reduces cellular penetrability of the fusions while keeping the specificity of the T22-CXCR4 interaction. All these data instruct how multifunctional nanoscale protein carriers can be designed for smart, protein-driven drug delivery, not only for the treatment of CXCR4+ human neoplasias, but also for the development of anti-HIV drugs and other pathologies in which CXCR4 is a relevant homing marker.
