https://www.sciencedirect.com/science/article/pii/S1742706118306299
Abstract
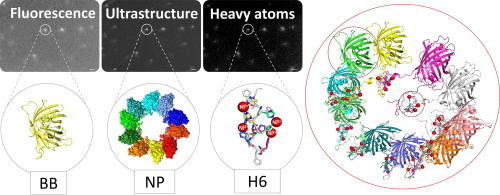
Nanostructured protein materials show exciting biomedical applications, since both structure and function can be genetically programmed. In particular, self-assembling histidine-rich proteins benefit from functional plasticity that allows the generation of protein-only nanoparticles for cell targeted drug delivery. However, the rational development of constructs with improved functions is limited by a poor control of the oligomerization process. By exploring cross-interactions between histidine-tagged building blocks, we have identified a critical architectonic role of divalent cations. The obtained data instruct about how histidine-rich protein materials can be assembled, disassembled and reassembled within the nanoscale through the stoichiometric manipulation of divalent ions, in a biochemical approach to biomaterials design.
Graphical abstract
Statement of Significance
Divalent metal and non-metal cations such as Ni2+, Cu2+ Ca2+ and Zn2+ have been identified as unexpected molecular tools to control the assembling, disassembling and reassembling of histidine-rich protein materials at the nanoscale. Their stoichiometric manipulation allows generating defined protein-protein cross-molecular contacts between building blocks, for a powerful nano-biochemical manipulation of the material’s architecture.
