Abstract
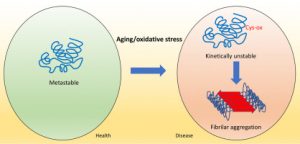
Oxidatively modified forms of proteins accumulate during aging. Oxidized protein conformers might act as intermediates in the formation of amyloids in age-related disorders. However, it is not known whether this amyloidogenic conversion requires an extensive protein oxidative damage or it can be promoted just by a discrete, localized post-translational modification of certain residues. Here, we demonstrate that the irreversible oxidation of a single free Cyssuffices to severely perturb the folding energy landscape of a stable globular protein, compromise its kinetic stability, and lead to the formation of amyloids under physiological conditions. Experiments and simulations converge to indicate that this specific oxidation-promoted protein aggregation requires only local unfolding. Indeed, a large scale analysis indicates that many cellular proteins are at risk of undergoing this kind of deleterious transition; explaining how oxidative stress can impact cell proteostasis and subsequently lead to the onset of pathological states.