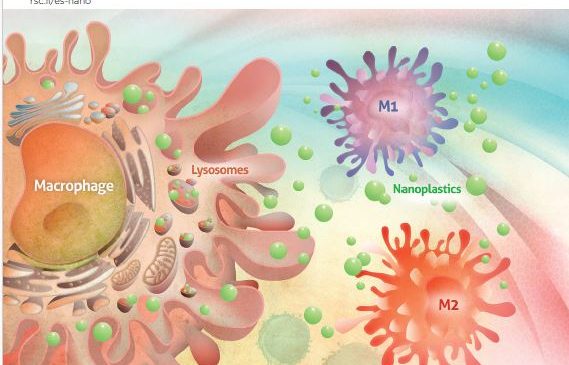
NANOLIGENT ha estat escollida per l’Associació d’Empreses de Cerdanyola del Vallès-Cerdanyola Empresarial per rebre el Reconeixement a la Innovació del 2023
El grup de Nanobiotecnologia de l'IBB-MRB, es complau en comunicar que NANOLIGENT ha estat escollida per l’Associació d'Empreses de Cerdanyola del Vallès-Cerdanyola Empresarial per rebre el Reconeixement a la Innovació del 2013. El Reconeixement va ser lliurat a la CEO de l'empresa, la Sra. Montserrat Cano, el 23 de novembre de 2023 a les 20 h a l’Hotel Campus de la UAB, en el marc de la X Nit de Cerdanyola Empresarial. Aquest Reconeixement premia les seves activitats en el desenvolupament de nous fàrmacs antimetastàsics que duem a terme a través del projecte RETOS COLABORACIÓN que està actualment actiu (New protein-based nanodrugs for the development of targeted tumor-agnostic therapy, amb referència CPP2021-008946). Aquest projecte involucra el nostre grup i el grup dirigit pel Prof. Ra