Perfecting prediction of mutational impact on the aggregation propensity of the ALS-associated hnRNPA2 prion-like protein
Cristina Batlle, María rosario Fernández, Valentín Iglesias, Salvador Ventura
http://onlinelibrary.wiley.com/doi/10.1002/1873-3468.12698/full
Abstract
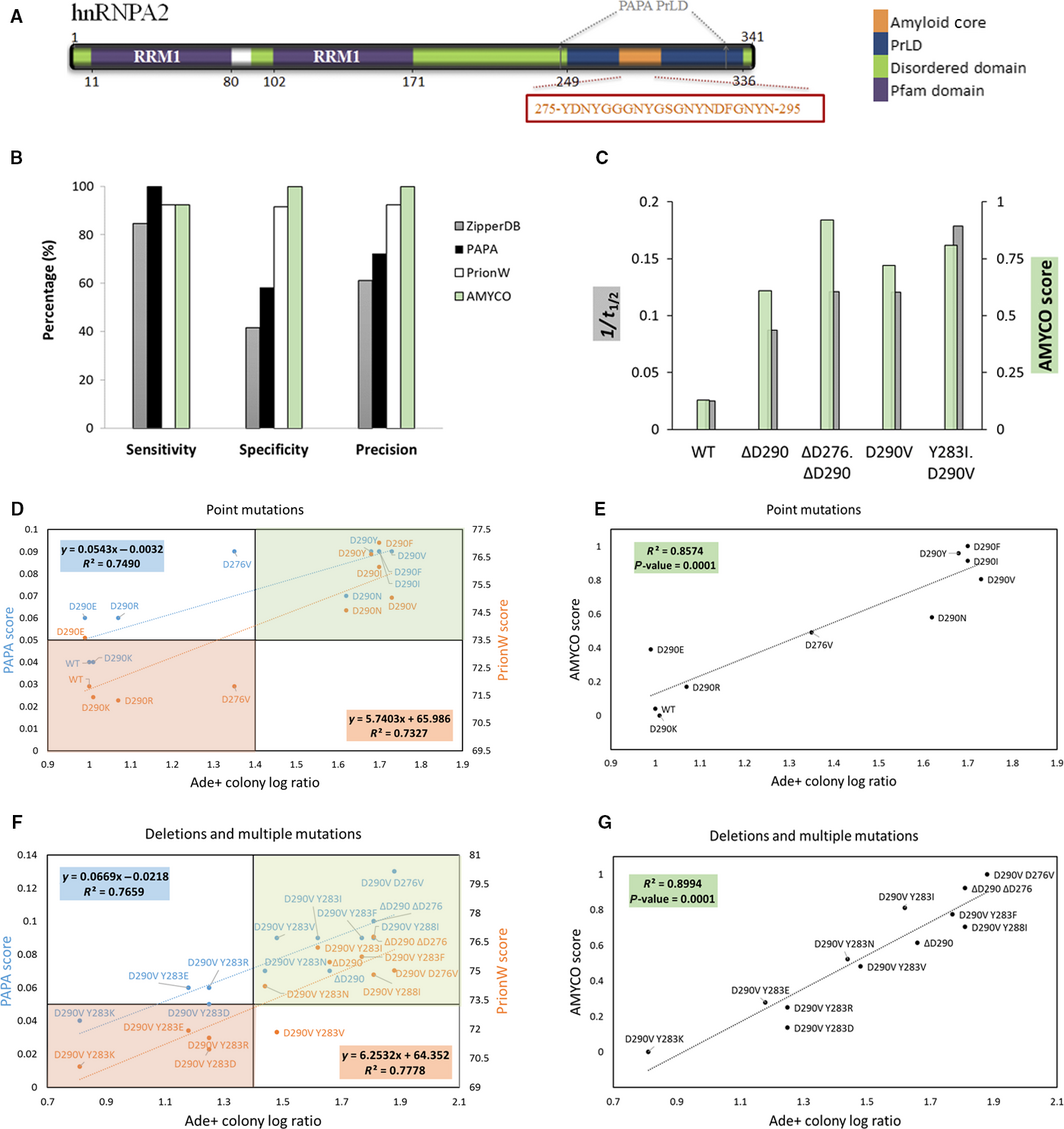
An increasing number of human proteins are being found to bear a prion-like domain (PrLD) driving the formation of membraneless compartments through liquid–liquid phase separation. Point mutations in these PrLDs promote the transition to an amyloid-like state. There has been much debate on whether this aberrant aggregation is caused by compositional or sequential changes. A recent extensive mutational study of the ALS-associated prion-like hnRNPA2 protein provides a framework to discriminate the molecular determinants behind pathogenic PrLDs aggregation. The effect of mutations on the aggregation propensity of hnRNPA2 is best predicted by combining their impact on PrLD amino acid composition and sequence-based amyloid propensity. This opens an avenue for the prediction of disease causing mutations in other human prion-like proteins.